Computer System Validation
We assist in ensuring that what you possess meets necessary qualifications and is validated
Computerized systems used for GxP processes, such as production, receiving raw materials, storage, dispensing, shipping finished products, quality control, etc., can affect product quality, data accuracy, and patient safety. Ideolon uses a risk-based approach to ensure GxP Computerized Systems by following the ‘V-Model’ from GAMP 5. This approach covers planning, specifications, setup, checking, and reporting, including tasks like confirming IT infrastructure, regular reviews, and closing systems.
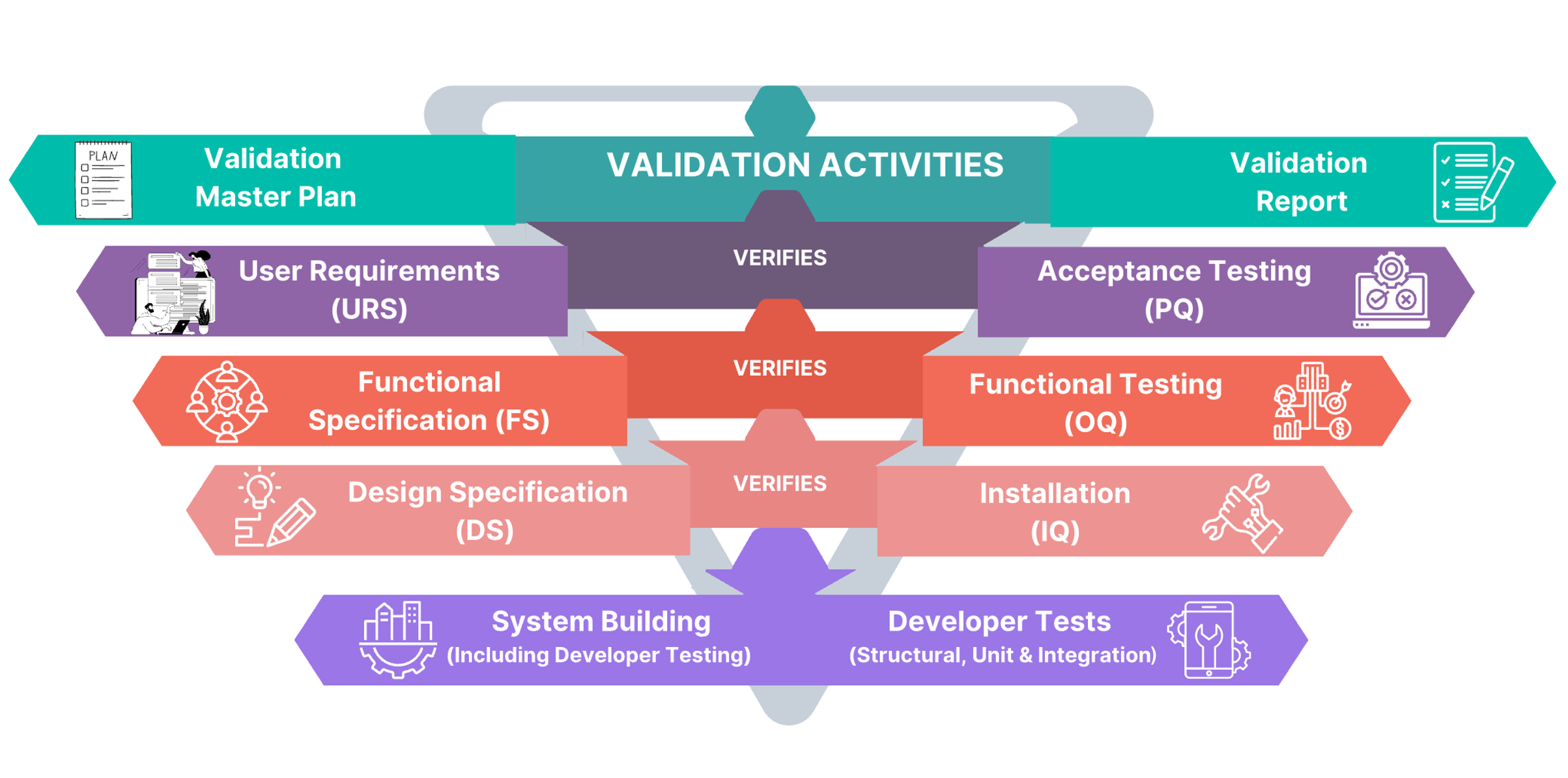
We are specialized in validating below systems
- Local Control System (SCADA, PLC)
- Plant Control System (SCADA, PLC)
- Data Integrity Risk Assessment
- Computer System Validation
- Building Management System, Environment management system
- Manufacturing Equipment
- CAPA System
- Automated Packaging & Labeling Equipment
- Track & Trace system
- Sterilization System
- Laboratory Systems (LIMS: Stand-alone & networked)
- Management Systems (ERP, SAP)
- Document Management System
- Warehouse Management System
- E-BMR System
- Sterilization System
What we bring to customer

Cost Saving & Time Optimization
Implementing correct CSV and CSA practices to ensure timely compliance and reduction of operational expense.

Committed To Compliance
Practicing 21 CFR part 11 and Annex 11 Compliance with clear evidence and audit trails.

Confidence
Our process instills confidence in accuracy, reliability, and consistency while preserving data integrity. We prioritize delivering trustworthy and dependable results for our customers.
Hybrid Execution Models
We offer flexible delivery models through Onsite, Remote and Offshore resources.
